Medical abortion treatment at home

For women from England and Wales. If you are from Scotland please click here
This service is a safe and legal way to end a pregnancy before 10 weeks gestation without needing to attend a clinic for treatment. You will have a telephone consultation and full medical assessment with a trained nurse or midwife who will assess your suitability for treatment. If treatment is suitable and safe, you will receive abortion pills by post a few days later, or we can book you for an alternative treatment in clinic. Sometimes a scan or other in-clinic checks are required before treatment.
Before your treatment
- If you are receiving your treatment pack by post, you will receive a box labelled Medabon, which contains 1 tablet of mifepristone (step 1) and 4 tablets of
misoprostol (step 2) - OR if you are receiving your treatment pack from a clinic, you will receive separate boxes of medication labelled mifepristone, containing 1 tablet (step 1), and misoprostol, containing 4 tablets (step 2)
- 2 additional misoprostol tablets to use if needed (step 3)
- Pregnancy test (step 4)
- Contraception and STI test (if requested and suitable) - Blood affects the results of this test, so it is best to complete this before starting your treatment. Click for details.
If you are receiving your treatment pack by post, this will be sent direct from the pharmacy from 1 to 3 days from your telephone consultation (sometimes longer with Bank Holidays). If your package is delayed in the post for a few days you should still take the tablets as directed once they arrive. The package is plain with no indication of its contents, it will be tracked but not signed for.
If anything in your medical situation has changed since your consultation and medical assessment, please contact us before starting your treatment.
Mental health concerns
If you wish to talk to someone within BPAS about your decision to have an abortion, we offer emotional support if you’ve had an abortion or are considering one. To book, please call 0345 730 4030.
If you are having feelings of self-harm or suicidal thoughts, please call 111 or 999.
Telephone 999 for an ambulance if you experience any of the following:
- Fainting or loss of consciousness
- Severe allergic reaction
- Concern for heart attack or stroke
- Chest pain
- Seizures / fits (especially new onset or unrelenting)
- Extremely heavy bleeding.
Sepsis is extremely rare, but the signs of potential sepsis are:
- Slurred speech or confusion
- Breathing difficulties
- Passing no urine in a day
- Skin mottled or discoloured
- Extreme shivering or muscle pain
- It feels like you are going to die.
If you need to call 999 or have any severe side effects, do not take any more of the medicines we have provided to you until you have been assessed by a healthcare professional.
BPAS Aftercare line
(24 hours a day, 7 days a week):
If you have any questions, problems, are unsure or worried about your treatment, please call: 0300 333 68 28 (or +44 1789 508 210)
Reasons you might call Aftercare:
- You are worried about your bleeding
- You are worried about the level of pain you are experiencing, and would like advice on pain relief
- You have signs of an infection, which could be:
a. A fever of 38C or more (more than 24 hours after misoprostol)
b. Ongoing pain
c. Unpleasant smelling bleeding or discharge from your vagina
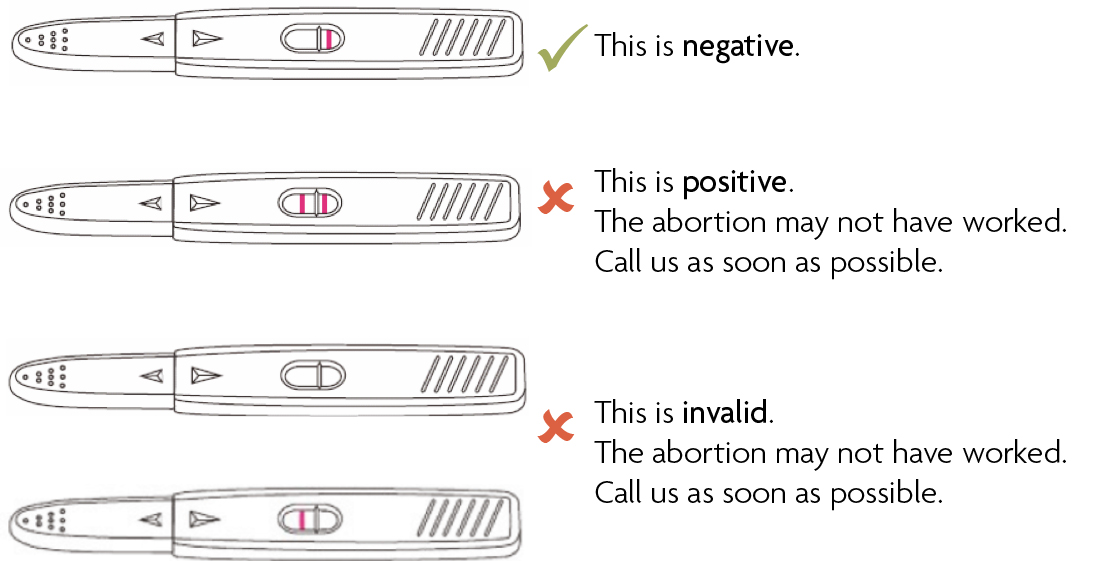
d. Pain or abdominal tenderness during sex - You take the pregnancy test that BPAS provided three weeks after your treatment (step 4) and it is positive, invalid, or you are unsure of your result.

We will explain the known risks and complications of your treatment during your telephone assessment. The risks and complications of this treatment are shown below.
This video is used to support your informed consent to EMA (Pills by Post) without ultra sound. It is a very informative video and by the end of it, you will understand the risks, benefits and alternatives to medical abortion without ultrasound. Many of our clients tell us watching this video is very valuable. It is 11 minutes long and cover all the things you need to consider when deciding on EMA pills by post without ultrasound.
Lower abdominal pain/cramping and vaginal bleeding are experienced by all people having medical abortion. Pain can be severe in some cases, requiring the use of strong painkillers (like codeine). Most abortions are complete within 4 hours of using misoprostol but for some it takes longer. Light vaginal bleeding or spotting can continue until the next menstrual period even if the abortion is complete.
Expected side effects of treatment include:
Nausea, vomiting (sickness), diarrhoea, headache, dizziness, fever/chills (1 in 10). If side effects continue after 24 hours of taking the second medication, please contact us.
If the side effects are severe or you have any of the symptoms for which you need to call 999, do not take any more of the medications we have provided to you until you are assessed by a healthcare professional.
These are usually easy to treat and rarely have any long-term health effects:
- Lower abdominal pain and cramping (experienced by all). Pain can be severe requiring strong painkillers
- Side effects of drugs such as nausea, vomiting, diarrhoea, headache, dizziness, fever/chills (1 in 10)
- Retained products of conception - where the pregnancy is no longer growing but some of the pregnancy tissue is left behind in the womb (2 in 100)
- Continuing pregnancy (less than 1 in 100)
- If you are treated without an ultrasound scan to date your pregnancy - the gestation of your pregnancy may be later than realised (less than 1 in 1,000). This can mean the abortion treatment fails, or there is more pain or bleeding, you may see a recognisable fetus or in extreme circumstances a live birth (1 in 10,000).
- Haemorrhage – very heavy bleeding (2 in 1,000)
- Unpredictable time to complete the procedure
- Unpredictable, irregular or prolonged bleeding after the abortion. Light bleeding or spotting can continue to next period (variable)
These risks may have long-term health effects:
- Undiagnosed ectopic pregnancy (2 in 1000)
- Infection of the womb or fallopian tubes (2 in 1,000)
- Blood clot in the lung or leg (VTE) (3 in 10,000)
- Psychological problems (variable)
Extra procedures that may be necessary
- Surgical abortion or uterine aspiration (1-2 in 100)
- Blood transfusion (2 in 1000)
- Laparoscopy or laparotomy – operation to look inside the abdomen (3 in 100,000)
- Hysterectomy – surgical removal of the womb (2 in 100,000)
- Intravenous antibiotics (2 in 10,000)
- Salpingectomy (removal of fallopian tube) or salpingostomy (opening the fallopian tube) (1 in 10,000)
Death is very rarely linked to abortion treatment (less than 1 in 100,000 for all abortions).
Treatment
The abortion pill involves taking two medicines, mifepristone and misoprostol.
The first medicine, mifepristone, ends the pregnancy. It works by blocking the hormone progesterone. Without progesterone, the lining of the uterus breaks down and the pregnancy cannot continue.
The second medicine, misoprostol, makes the womb contract causing cramping, bleeding and loss of the pregnancy similar to a miscarriage.
If at any point you decide to continue the pregnancy after taking the
medications, it is important to know that there could be risks to the developing fetus. Misoprostol can cause birth defects and both mifepristone and misoprostol can cause miscarriage. Contact BPAS for further information and advice.
For more detailed information about taking your medication, you can also watch our step by step videos.
The timing when you administer your medication is important. If your pregnancy is of 10 weeks gestation you will need to come into the clinic to take your medications.
You can also watch our step by step videos to help you take your medication.
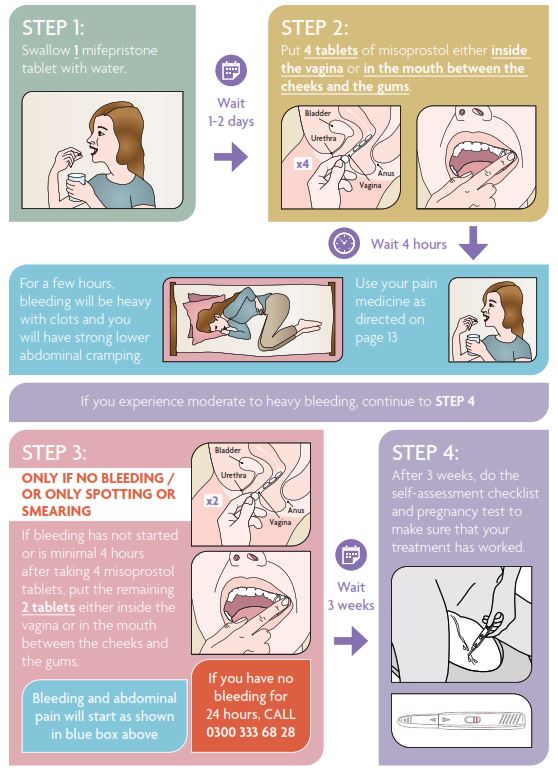
Step 1 – Mifepristone
Swallow the mifepristone tablet with water.
You may have nausea or vomiting after swallowing the mifepristone. If you do vomit, you should still use the misoprostol as outlined below.
Please telephone if you have any concerns or if you vomit within 30 minutes of swallowing the mifepristone.
Most women do not have bleeding or pain until they take the second medication (misoprostol). Bleeding can occur after taking mifepristone but it is usually light.
If you have started bleeding after step 1, you should still use the misoprostol (step 2), but placing the misoprostol in your mouth is preferred (placing between cheek and gum).
If you place these tablets between your cheek and gum, you are more likely to experience nausea and diarrhoea. Contact us if you have any concerns.
Step 2 and 3 – Misoprostol (to be taken 1-2 days after the mifepristone in Step 1)
Misoprostol tablets are placed in the vagina or between the cheek and gum. See below for more detail.
Misoprostol (the second medication) causes strong, painful cramps and heavy bleeding.
Step 2 – misoprostol
You have 6 tablets of misoprostol in total.
Use 4 tablets placed either in your vagina or between your cheek and gum.
Keep the other 2 tablets safe for step 3.
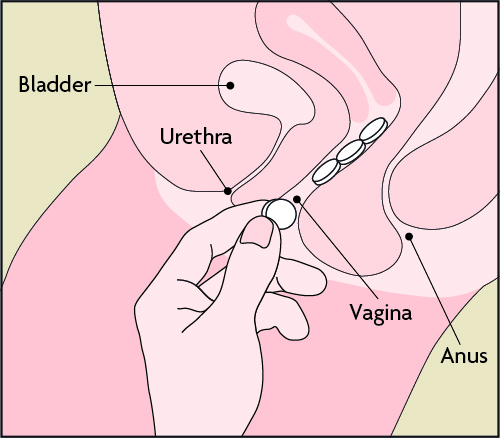
Into the vagina
Insert 4 tablets as high as possible in the vagina; the exact location is not important only that they do not fall out. We recommend doing this lying down to avoid the tablets falling out, but you can do this whilst, squatting or standing with one leg up - whatever is most comfortable for you.
The tablets should remain inside your vagina for 30 minutes. You could lie down for 30 minutes after insertion. Don’t worry if they fall out after this time or you see them pass once you start bleeding, it’s just the chalky residue of the medication, as long as they remain inserted for 30 minutes.
OR
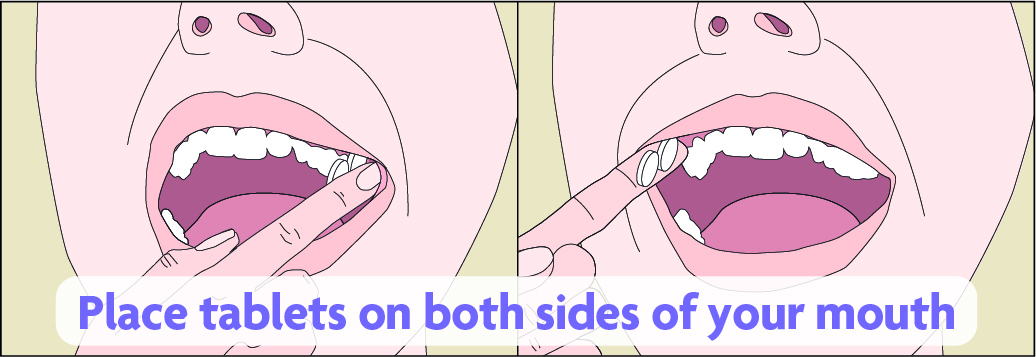
Into the mouth between cheek and gum
Place 4 misoprostol tablets into the mouth between the upper cheek and gum and allow the tablets to dissolve for 30 minutes. If the tablets have not completely dissolved within 30 minutes, you may swallow what is left with water.
Some people describe the taste of misoprostol as unpleasant and the texture as chalky. Placing the tablets between the cheek and gum is associated with higher rates of nausea, vomiting and diarrhoea.
Step 3 – additional misoprostol if needed
If 4 hours have passed and vaginal bleeding has not started or is minimal (meaning only spotting or smearing - only seeing blood on a tissue when wiping with a tissue), use the additional 2 misoprostol tablets either inside the vagina or between the cheek and gums.
Use the bleeding pictures in the "Bleeding" section below to help assess your bleeding.
Please note the foil on the reverse of these tablets is marked with a warning that says ‘Do not use in pregnancy’. This is because misoprostol can cause miscarriage which is why it is used in abortion care.
The abortion pill causes bleeding and pain that can last several hours or more. Pain usually begins 1-2 hours after misoprostol tablets, sometimes sooner. You can listen to descriptions of pain from real experiences on our Experiences of pain during an early medical abortion page.
Bleeding usually starts within 2 to 4 hours after using the misoprostol tablets. Please refer to the ‘Bleeding’ section below for more information about bleeding.
For some people, pain and bleeding can start more quickly but for others it can take longer. Pain and bleeding are often greatest when the pregnancy is being expelled. Most women pass the pregnancy within 4 hours of using the misoprostol however timings vary; and almost all women pass the pregnancy in the first 24 hours.
People have different experiences of pain. Some will experience intense pain, but some will only have mild pain. You can listen to descriptions of pain from real experiences on our Experiences of pain during an early medical abortion page.
There are some things that you can do to help reduce the pain you experience:
- Wear comfortable and loose clothing
- Stay in a familiar and relaxing place
- Apply a heating pad or hot water bottle to your lower stomach
- Take pain relief medicines. Please read the following guidance for ibuprofen,
paracetamol and codeine.
Take IBUPROFEN (also known as Brufen® or Nurofen®)
Ibuprofen is the best pain relief option as it targets the cause of pain. You should take ibuprofen unless you have been told not to by a healthcare professional.
Where to get ibuprofen:
It can be bought from most shops and supermarkets as well as pharmacies.
How to take ibuprofen:
Take 800mg when you start to feel pain after using step 2 (misoprostol).
Check which strength tablets you have:
- Take either 4 x 200mg
- Or 2 x 400mg
Every 8 hours if you need to.
Note: This dose is higher than recommended on the box, but is the best dose
for helping pain caused by taking abortion pills.
Safety warning: Do not take more than 2,400mg of Ibuprofen in 24 hours. (That
is 12 x 200mg tablets or 6 x 400mg tablets).
Take PARACETAMOL
You may choose to take paracetamol to help with your pain relief.
Where to get paracetamol:
It can be bought from most shops and supermarkets as well as pharmacies.
How to take paracetamol:
Take 1,000mg when you start to feel pain after using step 2 (misoprostol):
Take 2 x 500mg tablets
Every 4 hours if you need to.
Safety warning: Do not take more than 8 paracetamol tablets in a day (4,000mg
in 24 hours).
Do not take with any other paracetamol containing products (co-codamol, Lemsip®, cold and ‘flu tablets).
Codeine
Most people should not take codeine as the side effects can be harmful and it doesn’t work well for the type of pain experienced when taking abortion pills.
Codeine may be offered as an option after discussion at your appointment if you cannot use other options.
Where to get codeine:
Co-codamol can be taken if you are unable to take Ibuprofen. This can be purchased from pharmacies if needed.
Alternatively, codeine could be prescribed for you and included in your pack if it’s agreed that codeine is right for you and needed.
How to take codeine/co-codamol:
Take as directed on the pack.
Pain that you can’t tolerate after using pain relief
You should telephone BPAS on 0300 333 68 28 for advice if you are experiencing pain that you can’t tolerate after using pain relief.
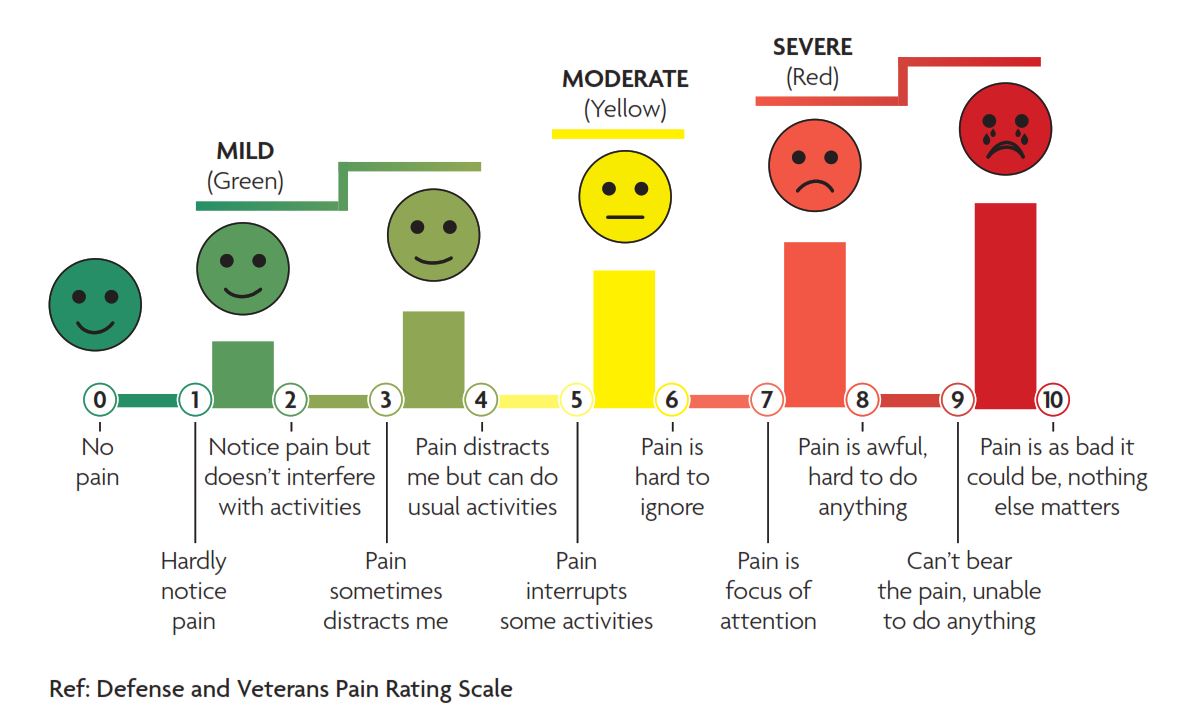
We will ask you how you score your pain out of 10. Please use the scale below and pay close attention to the words under the pain scores.
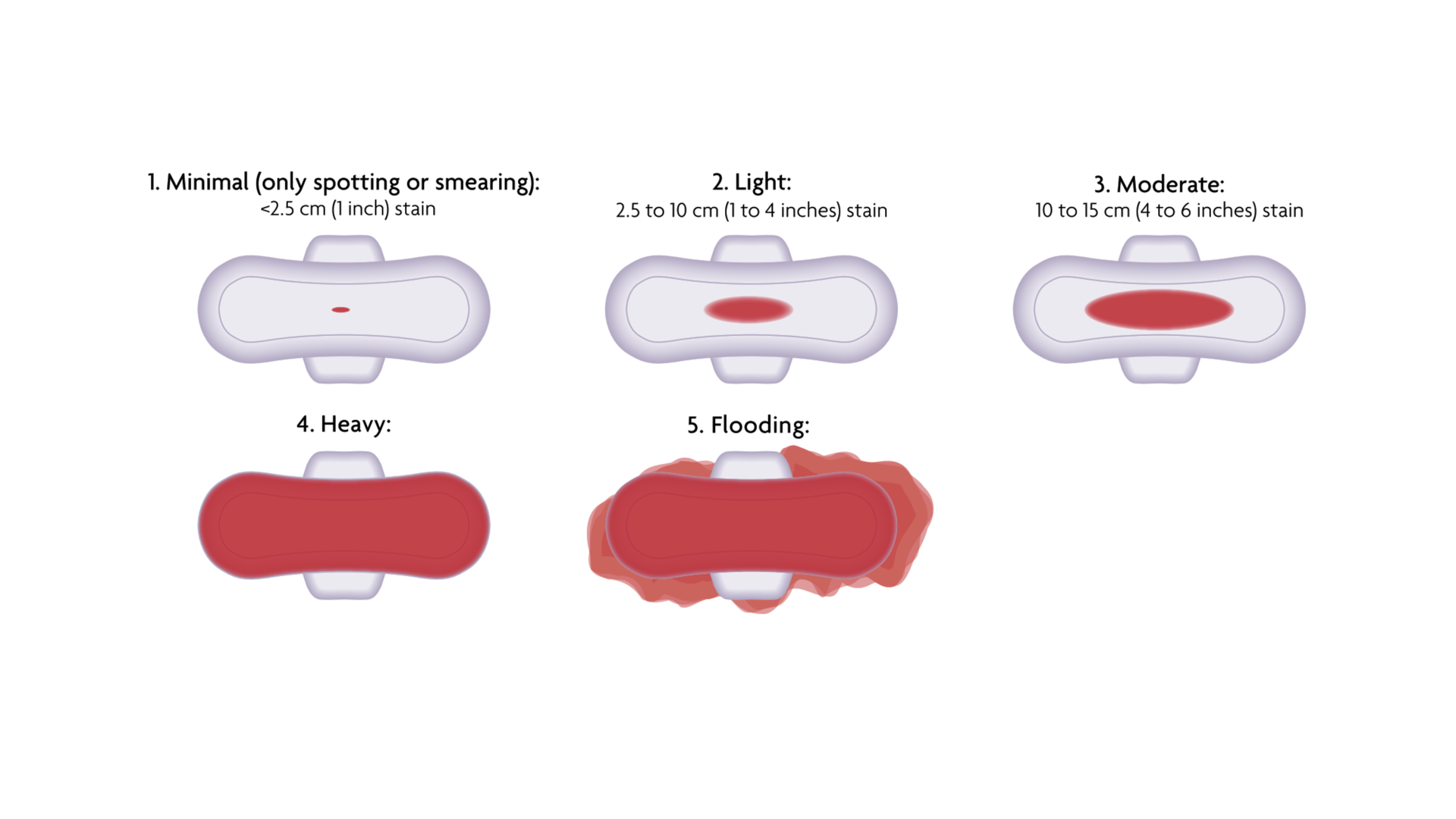
Use sanitary towels (maternity pads or thick overnight pads) to monitor your bleeding during abortion pill treatment. The amount and type of bleeding can vary for each person and each abortion. Bleeding is usually moderate to heavy but for some women it can be light.
You should telephone BPAS on 0300 333 68 28 for advice if:
- 24 hours after taking the misoprostol, you do not bleed at all, have spotting / only see blood on a tissue when wiping (see Minimal image 1)
- You experience flooding or heavy bleeding soaking 2 maternity pads or thick overnight pads for 2 hours in a row (see Heavy image 4)
Call 999 if you experience very heavy bleeding (see Flooding image 5) and feel unwell
Prolonged moderate to heavy bleeding can lead to significant blood loss and anaemia so please get in touch if this occurs.
Not everyone will pass blood clots during a medical abortion, but for those who do, the clots should be no larger than a lemon. You may see other tissue when you pass the pregnancy. This is larger and more recognisable at higher gestations, but in most cases the fetus cannot be seen without magnification.
Once the pregnancy passes, the amount of bleeding and pain should noticeably reduce. It is likely you will feel cramping on and off for a week or so but this should be easily managed with ibuprofen or paracetamol. Most women have light bleeding for about 2 weeks, but you may have spotting up to your next period. Sometimes you may have a short episode of pain with a gush of blood or a clot several weeks after the abortion - contact us if this continues.
Since you will pass the pregnancy at home, you can decide how you wish to dispose of the pregnancy remains. They can be flushed down the lavatory or wrapped in tissue, placed in a small plastic bag and put in the dustbin.
If you have any other questions about the disposal of the pregnancy remains, please click here.
After your treatment
The abortion pill is very effective and usually uncomplicated, but it is important to make sure it has worked. Having some cramping and bleeding does not guarantee that your treatment was successful. Misoprostol may cause serious birth defects if the pregnancy continues. If the abortion pill does not work for you, you should contact us to discuss your options.
- You will NOT be contacted by BPAS to find out if your treatment has worked (unless we have discussed this with you during your consultation)
- You need to complete the ‘self-assessment checklist’ below to ensure your treatment has worked and that you are no longer pregnant
- 3 weeks after starting your treatment, you should use the pregnancy test we sent you, with the first urine you passed when you woke up in the morning. (see below)
You must use the pregnancy test from your treatment pack for your 3 week urine test.
I will contact BPAS if I experience any of the following signs that my treatment has not worked:
- I did not bleed (or had only spotting, or only saw blood on a tissue when wiping) within 24 hours of taking misoprostol tablets
- I had less than 4 days of bleeding
- By the end of week 1, I still ‘feel’ pregnant or have symptoms of pregnancy such as sore breasts, sickness, tummy growing, etc.
- 3 weeks after treatment, I performed the BPAS urine pregnancy test (using the first urine passed when I woke) and the test was positive, invalid, or I was not sure of the result
- My next period has not come by 4-6 weeks after treatment (even if the pregnancy test was negative)
Telephone BPAS immediately on 0300 333 68 28 (or +44 1789 508 210) if you experience any signs listed above.
Pregnancy test instructions
You must use the pregnancy test from your treatment pack for your 3 week urine test.
The pregnancy test we sent with your medication should be used 3 weeks after you swallow the first medication (mifepristone). The pregnancy test should be performed using the first urine you pass after waking in the morning.
This is a diagram of your pregnancy test:
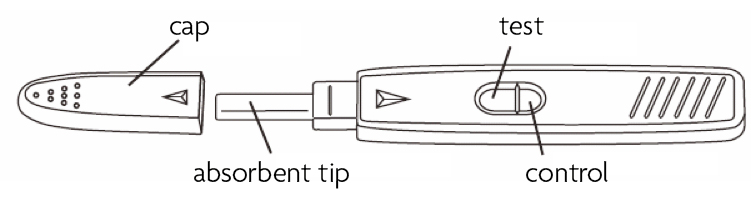
- Remove all packaging
- Remove the cap from the test
- As you urinate, hold the absorbent tip of the test in the urine stream or collect some urine in a clean pot and dip the tip into the urine for 5 to 10 seconds
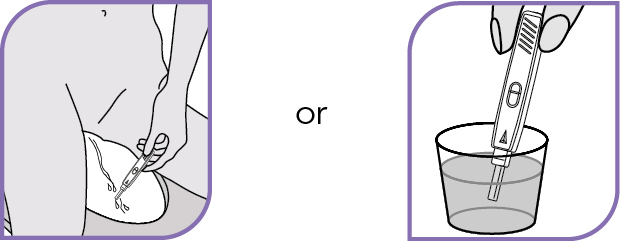
- Wait 5 to 10 minutes
- Read the pregnancy test
Our Aftercare Line is open 24 hours a day, 7 days a week. If you have any questions or are unsure about anything, please call 0300 333 68 28 (or +44 1789 508 210) for help.
What to expect after treatment
Recovery after an uncomplicated abortion usually happens fairly quickly, but it is different for every woman. There are some things to expect, which are normal, and other signs and symptoms that are not. It’s important that you know about both.
Most women recover quickly after an abortion. How much pain and bleeding you experience afterwards can vary.
If you’ve been prescribed blood thinning medication
After assessment, you may meet the criteria to be prescribed blood thinning injections. The process of injecting these and when to use them will be explained to you during a consultation.
If it is time to start taking your blood thinners, but your bleeding is heavy (see
"Bleeding" section above for image of heavy bleeding), do not use it. Wait another 24 hours to see if bleeding improves. If you continue to experience heavy bleeding 48 hours after using misoprostol, you should call us on 0300 333 68 28 (or +44 1789 508 210).
Feelings
After an abortion, most women feel relieved, but some may also feel sad or guilty.
If you feel you need to talk to someone, you can call us on 03457 30 40 30 (or +44 1789 508 211) to make an appointment for post-abortion counselling. This is a free service for women who have had treatment at BPAS.
Physical symptoms as your body recovers
Symptoms of nausea, vomiting and tiredness usually stop within 3 days of an abortion. Sore breasts may last 7 to 10 days. Take ibuprofen or paracetamol if necessary for the pain.
Mental health concerns
If you wish to talk to someone within BPAS about your decision to have an abortion, we offer emotional support if you’ve had an abortion or are considering one. To book, please call 0345 730 4030.
If you are having feelings of self-harm or suicidal thoughts, please call 111 or 999.
Contact our Aftercare Line immediately on 0300 333 68 28 (or +44 1789 508 210) if:
• you have heavy vaginal bleeding and have soaked through 2 or more large maxi pads an hour, for the last 2 hours
• it is more than 24 hours since you took your misoprostol and you still feel sick, have abdominal discomfort, diarrhoea, nausea, vomiting or weakness
• you have abdominal pain or discomfort that is not helped by medication, rest, a hot water bottle, or a heating pad
• you have a fever of 38°C or higher
• you have an unpleasant smelling discharge from your vagina
• if treated without a scan and you experience any of the following:
- no bleeding or only spotting/smearing 24 hours after using misoprostol
- lower abdominal pain worse on one side
- light-headedness, dizziness or feeling faint
- shoulder tip pain
• if you were treated following a scan showing a pregnancy in your uterus and you have no bleeding or only spotting/smearing 5-7 days after using
• if you have a positive pregnancy test or other sign listed on the self-assessment checklist
Telephone 999 for an ambulance if you experience any of the following:
• Fainting or loss of consciousness
• Severe allergic reaction
• Concern for heart attack or stroke
• Chest pain
• Seizures / fits (especially new onset or unrelenting)
• Extremely heavy bleeding.
Sepsis is extremely rare, but the signs of potential sepsis are:
• Slurred speech or confusion
• Breathing difficulties
• Passing no urine in a day
• Skin mottled or discoloured
• Extreme shivering or muscle pain
• It feels like you are going to die.
If you need to call 999 or have any severe side effects, do not take any more of the medicines we have provided to you until you have been assessed by a healthcare professional.
More information
Bathing
You can take a bath or shower as normal.
Sex
You can have sex as soon as you feel sufficiently recovered from the abortion. You can get pregnant almost immediately after treatment, so it is important to use contraception if you do not want to get pregnant.
Other activities, including work
Most women are fit and well enough to return to normal activities within a day or two. Rest until you feel ready to return to your normal routine.
Does having an abortion increase my chance of getting breast cancer?
No, there is no proven association with breast cancer and abortion.
Can I continue breastfeeding during medical abortion?
Both mifepristone and misoprostol pass into the breast milk but the amounts are small and should not cause any adverse effects in breastfed infants. Breastfeeding may continue uninterrupted following mifepristone and misoprostol. If breastfeeding an infant under 6 months’ of age, express and discard milk whilst taking codeine and for the first feed after the last dose of codeine.
Will having an abortion cause problems with future pregnancies?
If your abortion is uncomplicated, there should be no issues with future pregnancies as a result of the abortion.
There are no proven associations between abortion and future infertility, ectopic pregnancy or placenta praevia.
Having an abortion may be associated with future pregnancies ending before the due date. This risk appears to increase when someone has had more than one abortion.
How will I feel afterwards?
Every woman is different so they feel, experience and cope in a way which is individual to them. There is no evidence to suggest that you will experience any mental health issues as a result of an abortion. However, if you have a history of mental health problems you may still have those problems whether you choose to have the abortion or not.
What about travel after treatment?
Travel is not ideal in the first 24 hours after using misoprostol. You may experience symptoms whilst in transit. You will need to know how to access emergency services in case of a complication.
What about contraception and STI screening?
Getting tested for STIs is recommended if you are at risk of infection. Almost all methods of contraception can be started on the same day as medical abortion or the day after. If you choose intrauterine contraception (IUD or coil) this can be inserted as soon as the abortion is complete. If you live in England, Scotland or Wales, you can see your GP or local contraception and sexual health service to obtain contraception after treatment. BPAS can provide some contraceptive methods and this will be discussed during your consultation.
To locate your nearest NHS contraception service or STI clinic, visit the following websites:
England: NHS Health Services
Northern Ireland: Informing Choices NI
Scotland: NHS Inform Sexual Health Services
Wales: NHS Wales Local Services
If you want to provide any feedback about the service you have received, please email youropinioncounts@bpas.org
After treatment, we will email you with a link to complete our satisfaction survey, if you agreed that we may do so.
There may be times you want to feedback formally or raise a complaint with us.
Our leaflet titled ‘Feedback and complaints policy’ explains our feedback and complaints process.
To raise a complaint you can:
- speak to a member of staff
- call 0345 365 50 50 (or +44 1789 508 211) and ask to speak to the Client Engagement Manager. If you are deaf or hard of hearing and use a textphone you can call 0345 365 1450 (or +44 1789 416 584)
- write to the Client Engagement Manager, British Pregnancy Advisory Service, Orion House, 2 Athena Drive, Tachbrook Park, Leamington Spa, CV34 6RQ, or email youropinioncounts@bpas.org
Abortion: Frequently asked questions
You might have lots of questions which we are happy to answer when you call or visit the clinic. We answer some of the things people ask and give links for more information below:
-
Can I have sex during the treatment?
-
You can have sex as soon as you feel sufficiently recovered from the abortion. You can get pregnant almost immediately after treatment, so it is important to use contraception if you do not want to get pregnant.
-
Can I have a bath?
-
You can take a bath or shower as normal.
-
Will I be able to work?
-
Most women are fit and well enough to return to normal activities within a day or two. Rest until you feel ready to return to your normal routine.
-
Is this funded by NHS?
-
Most of our patients (97%) have their treatment paid for either by the NHS or another government department. We need information about your location and GP to see if funding is available for you. Sometimes we may need to make a note of your NHS number (ask your GP Surgery if you don't have it)
-
How do I get the Pills?
-
You will need to have a consultation to ensure abortion treatment at home is legal, suitable and safe for you. We will explain the risks and complications of the treatment and ask for your consent. Most women are eligible for treatment at home and receive their treatment by post within 3 days. You can also choose to collect the medication from a BPAS clinic.
Need more help?
We are an independent healthcare charity which for more than 55 years, has been advocating and caring for women and couples who decide to end a pregnancy.